2025: A Year of Milestones in DS-AD
The medical advances made within the Down syndrome community over the past few decades have been nothing short of amazing. The life span of individuals with Down syndrome has more…
 2025: A Year of Milestones in DS-AD
2025: A Year of Milestones in DS-AD
 RAND report shows benefit of investment in future health outcomes for adults with Down syndrome
RAND report shows benefit of investment in future health outcomes for adults with Down syndrome
 Pathways to Parity Program Approved for $250K PCORI Engagement Award
Pathways to Parity Program Approved for $250K PCORI Engagement Award
 Down syndrome population will see a clinical trial of new Alzheimer’s drug
Down syndrome population will see a clinical trial of new Alzheimer’s drug
 Clinical Trials Day: A Historic Review of the First Clinical Trial
Clinical Trials Day: A Historic Review of the First Clinical Trial
 Down syndrome collaborators now published in Alzheimer’s and Dementia
Down syndrome collaborators now published in Alzheimer’s and Dementia
 LuMind IDSC statement on FDA Advisory Committee for promising Alzheimer’s drug, donanemab
LuMind IDSC statement on FDA Advisory Committee for promising Alzheimer’s drug, donanemab
 Study: Medicare, Medicaid, and Dual Enrollment for Adults with Intellectual and Developmental Disabilities.
Study: Medicare, Medicaid, and Dual Enrollment for Adults with Intellectual and Developmental Disabilities.
 Message from the CEO: a look ahead to 2024
Message from the CEO: a look ahead to 2024
 Boston Globe OpEd People with Down Syndrome Now Left Out of Treatment
Boston Globe OpEd People with Down Syndrome Now Left Out of Treatment
 Study: Use of Medicaid by Adults with Down Syndrome
Study: Use of Medicaid by Adults with Down Syndrome
 LuMind IDSC statement on FDA approval of Leqembi
LuMind IDSC statement on FDA approval of Leqembi
 Expert panel addresses inequitable access to Alzheimer’s drugs for adults with Down syndrome
Expert panel addresses inequitable access to Alzheimer’s drugs for adults with Down syndrome
 LuMind IDSC Statement re: Donanemab Phase 3 Results
LuMind IDSC Statement re: Donanemab Phase 3 Results
 Important progress in the treatment of sleep apnea and Down syndrome
Important progress in the treatment of sleep apnea and Down syndrome
 Hope and Action for Down syndrome Alzheimer’s research on WDSD 2023
Hope and Action for Down syndrome Alzheimer’s research on WDSD 2023
 Fierce Biotech article highlights dearth of inclusion in Alzheimer’s research
Fierce Biotech article highlights dearth of inclusion in Alzheimer’s research
 With support from Lilly, LuMind IDSC launches key research and awareness activities to advance Down syndrome research
With support from Lilly, LuMind IDSC launches key research and awareness activities to advance Down syndrome research
 Results from “Challenges of Caregiving” Survey Published in Research Journal
Results from “Challenges of Caregiving” Survey Published in Research Journal
 New Year Greetings from Hampus Hillerstrom
New Year Greetings from Hampus Hillerstrom
 Statement on accelerated approval of Leqembi to treat mild-stage Alzheimer’s disease in the general population
Statement on accelerated approval of Leqembi to treat mild-stage Alzheimer’s disease in the general population
 Impact Report 2022
Impact Report 2022
 New steps forward in treating Alzheimer’s disease with lecanemab: Clinical trial reveals clear efficacy in removing brain plaque
New steps forward in treating Alzheimer’s disease with lecanemab: Clinical trial reveals clear efficacy in removing brain plaque
 DS-CTN Data and Safety Monitoring Board (DSMB)
DS-CTN Data and Safety Monitoring Board (DSMB)
 Lecanemab: New Treatment for Alzheimer’s Disease
Lecanemab: New Treatment for Alzheimer’s Disease
 NTG Publishes Advisory on Long-COVID in Adults with Intellectual Disability
NTG Publishes Advisory on Long-COVID in Adults with Intellectual Disability
 Why is Down syndrome research important?
Why is Down syndrome research important?
 Potential New Therapy for Adults with Down Syndrome
Potential New Therapy for Adults with Down Syndrome
 LIFE-DSR: up close and personal with John
LIFE-DSR: up close and personal with John
 Caregivers Evaluate Independence in Individuals with Down Syndrome
Caregivers Evaluate Independence in Individuals with Down Syndrome
 Report of the Neuroatypical Conditions Expert Consultative Panel
Report of the Neuroatypical Conditions Expert Consultative Panel
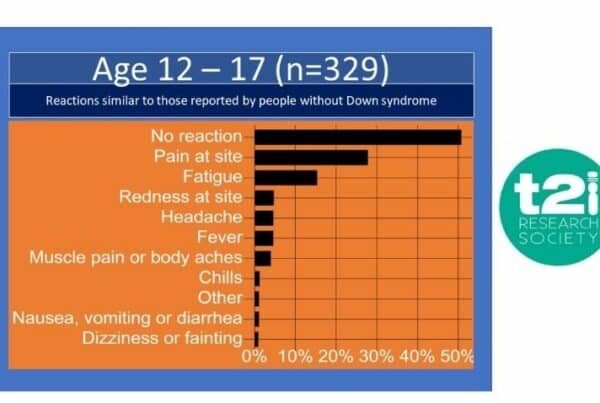 Results from T21RS’s COVID-19 International Survey on Vaccination of Individuals with DS
Results from T21RS’s COVID-19 International Survey on Vaccination of Individuals with DS
 Five Recent Down Syndrome Research Data Articles to Stay Up to Date
Five Recent Down Syndrome Research Data Articles to Stay Up to Date
 Publication Illustrates Need for Therapies to Address Immune Aging in Down Syndrome
Publication Illustrates Need for Therapies to Address Immune Aging in Down Syndrome
 LuMind IDSC Statement on CMS Proposed Coverage for Anti-Amyloid AD Drugs
LuMind IDSC Statement on CMS Proposed Coverage for Anti-Amyloid AD Drugs
 LuMind IDSC, NDSS and NTG Urge the Advisory Council to Take Action on the Needs of the DS Community
LuMind IDSC, NDSS and NTG Urge the Advisory Council to Take Action on the Needs of the DS Community
 LuMind IDSC and Dr. Nicole White Publish: Parental Perspectives on Research for Down Syndrome
LuMind IDSC and Dr. Nicole White Publish: Parental Perspectives on Research for Down Syndrome
 LuMind IDSC and Team of Experts Publish Article on Aging with DS and the Future of Research
LuMind IDSC and Team of Experts Publish Article on Aging with DS and the Future of Research
 Expert Chat: A Conversation with Dr. Nicole White
Expert Chat: A Conversation with Dr. Nicole White
 LuMind IDSC and Other National Groups Urge CMS to Include DS Community in Alzheimer’s Drugs Coverage
LuMind IDSC and Other National Groups Urge CMS to Include DS Community in Alzheimer’s Drugs Coverage
 A New Perspective on The Aduhelm Controversy
A New Perspective on The Aduhelm Controversy
 LuMind IDSC signs on to NTG statement re: Aduhelm
LuMind IDSC signs on to NTG statement re: Aduhelm
 Why Patient Voices Are So Critical to the Development and Initial Approval of New Drugs
Why Patient Voices Are So Critical to the Development and Initial Approval of New Drugs
 FDA Approves Biogen’s Aduhelm, the First New Drug for Alzheimer’s Disease Since 2003
FDA Approves Biogen’s Aduhelm, the First New Drug for Alzheimer’s Disease Since 2003
 Cross-Sectional Exploration of Plasma Biomarkers of Alzheimer’s Disease in Down Syndrome: Early Data from the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study
Cross-Sectional Exploration of Plasma Biomarkers of Alzheimer’s Disease in Down Syndrome: Early Data from the Longitudinal Investigation for Enhancing Down Syndrome Research (LIFE-DSR) Study
 LuMind IDSC Supported the T21RS COVID-19 Initiative’s International Survey on Patients with COVID-19 and Down Syndrome
LuMind IDSC Supported the T21RS COVID-19 Initiative’s International Survey on Patients with COVID-19 and Down Syndrome
 Findings Released for Ongoing Study of Clinical Trial Participation by the Down Syndrome Community
Findings Released for Ongoing Study of Clinical Trial Participation by the Down Syndrome Community
 LuMind IDSC is Excited About a New Publication in the Journal of Patient-Reported Outcomes
LuMind IDSC is Excited About a New Publication in the Journal of Patient-Reported Outcomes
 LuMind IDSC Acknowledged by T21RS For Work on COVID-19 Survey
LuMind IDSC Acknowledged by T21RS For Work on COVID-19 Survey
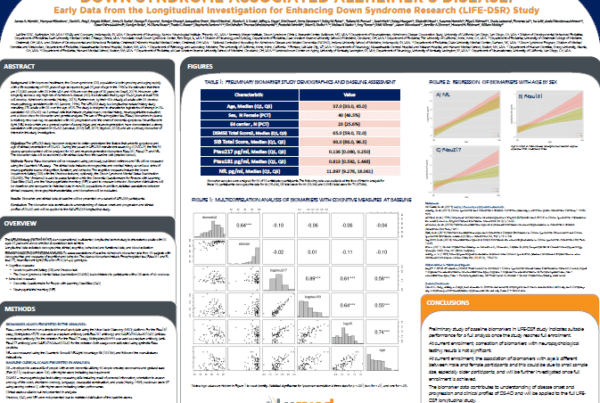 LIFE-DSR Natural History Study Blood Biomarker Data Shared at CTAD
LIFE-DSR Natural History Study Blood Biomarker Data Shared at CTAD
 myDSC Webinar – Fighting Alzheimer’s Disease in Down Syndrome: There is Hope!
myDSC Webinar – Fighting Alzheimer’s Disease in Down Syndrome: There is Hope!
 The LuMind IDSC Foundation Partners with Eli Lilly and Company to Measure Blood Biomarkers in LIFE-DSR Natural History Study
The LuMind IDSC Foundation Partners with Eli Lilly and Company to Measure Blood Biomarkers in LIFE-DSR Natural History Study
 LuMind IDSC and NDSS Submit Joint Recommendations for Advancing NIH-Funded Down Syndrome Research
LuMind IDSC and NDSS Submit Joint Recommendations for Advancing NIH-Funded Down Syndrome Research
 T21RS Publishes Initial Results of COVID-19 and Down Syndrome Survey and Seeks More Participants
T21RS Publishes Initial Results of COVID-19 and Down Syndrome Survey and Seeks More Participants
 National Down Syndrome Organizations Combine Efforts to Publish Q&A on COVID-19 and Down Syndrome
National Down Syndrome Organizations Combine Efforts to Publish Q&A on COVID-19 and Down Syndrome
 A Memory Drug for Down Syndrome?
A Memory Drug for Down Syndrome?
 $60M in NIH INCLUDE Funding for Down Syndrome Research in 2020
$60M in NIH INCLUDE Funding for Down Syndrome Research in 2020
 Clinical Trial Volunteers Give The Gift That Keeps On Giving
Clinical Trial Volunteers Give The Gift That Keeps On Giving
 Biogen Announces New Hope for Alzheimer’s Drug Candidate Aducanumab
Biogen Announces New Hope for Alzheimer’s Drug Candidate Aducanumab
 Clinicians Answer Additional Medical Questions From LuMind IDSC Research Rally Panel Q&A
Clinicians Answer Additional Medical Questions From LuMind IDSC Research Rally Panel Q&A
 LIFE-DSR Study – Why Natural History Studies Are Important on the Path to New Treatments
LIFE-DSR Study – Why Natural History Studies Are Important on the Path to New Treatments
 Cerveau Technologies, Inc. Announces Partnership with LuMind IDSC to Expand LIFE DSR Study
Cerveau Technologies, Inc. Announces Partnership with LuMind IDSC to Expand LIFE DSR Study
 LuMind Research Down Syndrome Foundation Launches the Down Syndrome Clinical Trials Network
LuMind Research Down Syndrome Foundation Launches the Down Syndrome Clinical Trials Network
 Published results on the Roche Clinical Trials for CLEMATIS expected in the first half of 2019
Published results on the Roche Clinical Trials for CLEMATIS expected in the first half of 2019
 New Trans-NIH INCLUDE Project for Down Syndrome will provide up to $261M Over 5 Years
New Trans-NIH INCLUDE Project for Down Syndrome will provide up to $261M Over 5 Years
 Historic ~65% increase for NIH Down syndrome research
Historic ~65% increase for NIH Down syndrome research
 Increased Commitment to Down Syndrome Research Included in 2018 Omnibus Spending Bill
Increased Commitment to Down Syndrome Research Included in 2018 Omnibus Spending Bill